Importance of the Extracellular Matrix (ECM)
- Structural support: The extracellular matrix provides physical support to cells and tissues, helping to maintain their shape, and keeping them in the proper position.
- Cell signaling and communication: The extracellular matrix plays a vital role in cell signaling and communication, regulating processes such as cell proliferation, differentiation, and migration.
- Tissue repair and regeneration: The extracellular matrix also plays a critical role in tissue repair and regeneration. When tissues are damaged, the ECM helps to create a scaffold for cells to migrate and proliferate, promoting tissue repair.
- Disease pathology: Abnormalities in the extracellular matrix can contribute to various disease pathologies, such as fibrosis, cancer, and cardiovascular disease. Understanding the role of the ECM in disease development and progression is crucial for developing effective treatments.
Extracellular Matrix (ECM) in Cancer
The extracellular matrix (ECM) plays a critical role in cancer development and progression. Abnormalities in the ECM can contribute to the formation of tumors, invasion, and metastasis of cancer cells.
- Tumor formation: The ECM provides a scaffold for cells to adhere to and grow, and abnormal changes in the ECM can promote tumor formation. For example, the ECM can become stiff, and this can promote the growth of cancer cells by signaling through specific receptors.
- Invasion and metastasis: Facilitates the invasion of cancer cells into surrounding tissues and promote the formation of metastases. Cancer cells can produce enzymes that degrade the ECM, allowing them to move more freely and invade other tissues.
- Chemoresistance: Contributes to chemoresistance by preventing drugs from reaching cancer cells by acting as a physical barrier that restricts the diffusion of drugs into the tumor.
- Immunotherapy resistance: Plays a significant role in immunotherapy resistance by creating a barrier that limits the effectiveness of immune-based treatments.
- Angiogenesis: Plays a role in angiogenesis, the process by which new blood vessels form. Cancer cells can produce factors that stimulate the formation of new blood vessels from the surrounding tissue, which can provide nutrients to the tumor.
Exploring the Boundless Applications of ECM in Immuno-Oncology
ECM, a dynamic and intricate network surrounding cells, has emerged as a key player in shaping the tumor microenvironment and influencing immune responses. In the field of immuno-oncology, the ECM offers a multitude of applications that hold promise for revolutionizing cancer treatment. By understanding the complex interplay between immune cells, tumor cells, and the ECM, we can unlock novel strategies to enhance anti-tumor immune responses and overcome the challenges posed by the tumor microenvironment.
- Modulation of immune cell behavior. The ECM provides critical signals that regulate immune cell recruitment, activation, and function within tumors. By deciphering these signals, we can develop targeted interventions to enhance immune cell infiltration, activation, and persistence, ultimately bolstering the body’s ability to recognize and eliminate cancer cells.
- Influencing the efficacy of immune checkpoint inhibitors: Understanding how the ECM affects checkpoint inhibitor responsiveness can guide the development of combination therapies that maximize treatment outcomes and overcome resistance mechanisms.
- Reshaping the tumor microenvironment: By targeting specific ECM components or remodeling enzymes, we can disrupt the immunosuppressive nature of the ECM, allowing immune cells to penetrate tumors more effectively and mount robust anti-tumor responses.
Challenges with Immunotherapy
Immunotherapy strategies are utilized to harness the power of the immune system to recognize and destroy cancerous cells. However key challenges remain. While immune checkpoint inhibitor therapy can provide a more effective therapeutic option for many different advanced stage cancers; approximately 30-40% of patients and nonspecific immunologic activation can lead to immune-related adverse events.
Next phase of cancer immunotherapy - Targeting the Tumor Stroma
Tumor development and progression are dependent on the cross talk between tumor cells, stromal cells, immune cells and the extracellular matrix (ECM). The TME consists of various cells such as myeloid derived suppressor cells (MDSCs), Tumor Associated Macrophages (TAMs) and Regulatory T cells (Tregs) and Cancer associated fibroblasts (CAFs) that contribute to immune cell dysfunction in the TME.
Tumor cells interact with the stroma and ECM leading to a remodeling of the tumor environment into a hypoxic and metabolically active state. In addition, tumors interact with immune cells promoting inflammatory responses that maintain tumor metastasis. Fibroblasts and other stromal cells in turn secrete cytokine responses to recruit immune cells thereby contributing to tumor metastasis.
In TME cancer cells directly interact with both the immune system and the stroma and in turn the stroma responds with pro-inflammatory and anti-tumor makers.
We aim to understand the interactions between stroma-cancer cells and immune system.
Importance of the Tumor Microenvironment
Tumor development and progression are dependent on the crosstalk between tumor cells, stromal cells, immune cells and the extracellular matrix (ECM). The TME consists of various cells such as myeloid derived suppressor cells (MDSCs), Tumor Associated Macrophages (TAMs) and Regulatory T cells (Tregs) and Cancer associated fibroblasts (CAFs) that contribute to immune cell dysfunction in the TME. In addition, studies have shown that T cells poorly migrate within the TME and are excluded from the tumor bed due to metabolic factors such as hypoxia and signaling pathways.
Understanding the cross talk among these various cell types in the progression of tumors has a major impact in developing therapies and identifying biomarkers for patient stratification. The advancements in imaging technologies and instrumentation have emphasized a greater role of the TME in contributing to the response of immunotherapies.
Classifications of the TME in response to current immunotherapies
Tumor development and progression relies on the cross talk among tumor cells, stromal and immune cells, the extracellular matrix and soluble cues. Over the past few years, increased data and advancements in imaging technologies have emphasized a greater role of the TME in contributing to the response of immunotherapies. This understanding has led to the classification of the TME as follows:
-
Immune inflamed profiles is characterized by the presence in the tumor core of cytotoxic T lymphocytes (CTL) which express PD-1 molecule along with PD-L1 positive tumor cells
-
Immune excluded profile is characterized by immune cells that remain in the stromal compartments of the TME and unable to migrate into the tumor bed
-
Immune-desert profile is characterized by the absence of immune cells or T cells and therefore resistance to immunotherapies such as PD-1
Since 2015, Screen Therapeutics has identified novel mechanisms and broad spectrum applications for known immunomodulatory targets.
Modules in IO
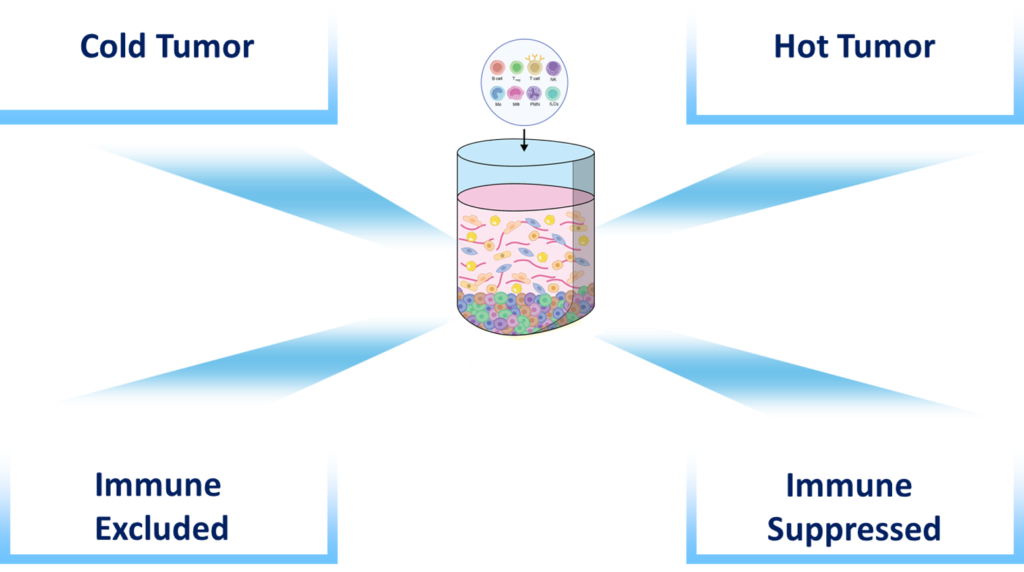
- Immune 3D can be used to assess factors that increase immune cell migration and infiltration, reduce the production of inhibitory factors and the recruitment of suppressive cells, modulate cellular adhesion, integrins, chemokines and nutrient availability.
- Immune 3D can be used to assess factors that increase immune cell migration and infiltration, reduce the production of inhibitory factors and the recruitment of suppressive cells, modulate cellular adhesion, integrins, chemokines and nutrient availability.
- Our system can be used to assess factors modulating immune cell function, such as immune checkpoints or inhibitor receptor expression.
- Spatial/ temporal relationships between immune cells, tumor cells and surrounding stroma.
- Immune 3D can be set in the presence and absence of immune cells providing a reproducible and affordable platform (compared to in vivo systems) to assess the impact of specific compounds on the tumor microenvironment and the relative contribution of the immune system.
- Importantly, the impact of specific compounds on human tumors and human immune cells can be observed and characterized.
- Immune 3D can be used in combination with immune suppressive agents such as chemotherapy, pharmacological inhibitors, as well as with cells that have been genetically manipulated in order to elucidate the mechanisms impacting anti-tumor immunity.
- Our system provides a novel platform to assess synthetic lethality and the impact of targeting defective DNA repair in cancer cells on anti-tumor immunity.
