Challenges with Immunotherapy
Importance of the Tumor Microenvironment
Tumor development and progression are dependent on the crosstalk between tumor cells, stromal cells, immune cells and the extracellular matrix (ECM). The TME consists of various cells such as myeloid derived suppressor cells (MDSCs), Tumor Associated Macrophages (TAMs) and Regulatory T cells (Tregs) and Cancer associated fibroblasts (CAFs) that contribute to immune cell dysfunction in the TME. In addition, studies have shown that T cells poorly migrate within the TME and are excluded from the tumor bed due to metabolic factors such as hypoxia and signaling pathways.
Understanding the cross talk among these various cell types in the progression of tumors has a major impact in developing therapies and identifying biomarkers for patient stratification. The advancements in imaging technologies and instrumentation have emphasized a greater role of the TME in contributing to the response of immunotherapies.
Classifications of the TME in response to current immunotherapies
Tumor development and progression relies on the cross talk among tumor cells, stromal and immune cells, the extracellular matrix and soluble cues. Over the past few years, increased data and advancements in imaging technologies have emphasized a greater role of the TME in contributing to the response of immunotherapies. This understanding has led to the classification of the TME as follows:
-
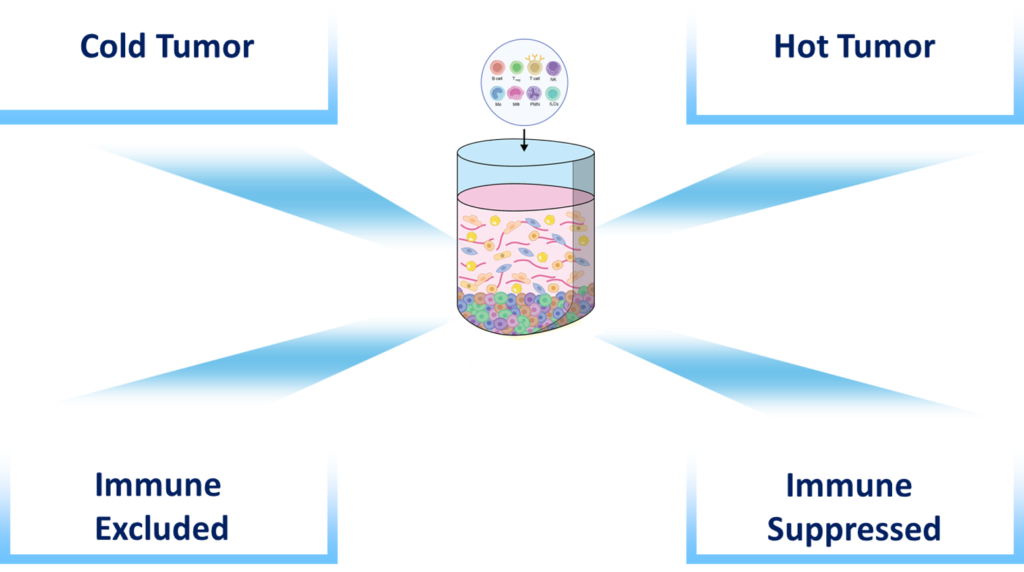
Immune inflamed profiles is characterized by the presence in the tumor core of cytotoxic T lymphocytes (CTL) which express PD-1 molecule along with PD-L1 positive tumor cells
-
Immune excluded profile is characterized by immune cells that remain in the stromal compartments of the TME and unable to migrate into the tumor bed
-
Immune-desert profile is characterized by the absence of immune cells or T cells and therefore resistance to immunotherapies such as PD-1
What are the sub-classes of TIME?
Lack of Tumor Infiltrating Lymphocytes (TIL) possibly due to factors such as tumor antigen suppression. The majority of tumors express this phenomenon.
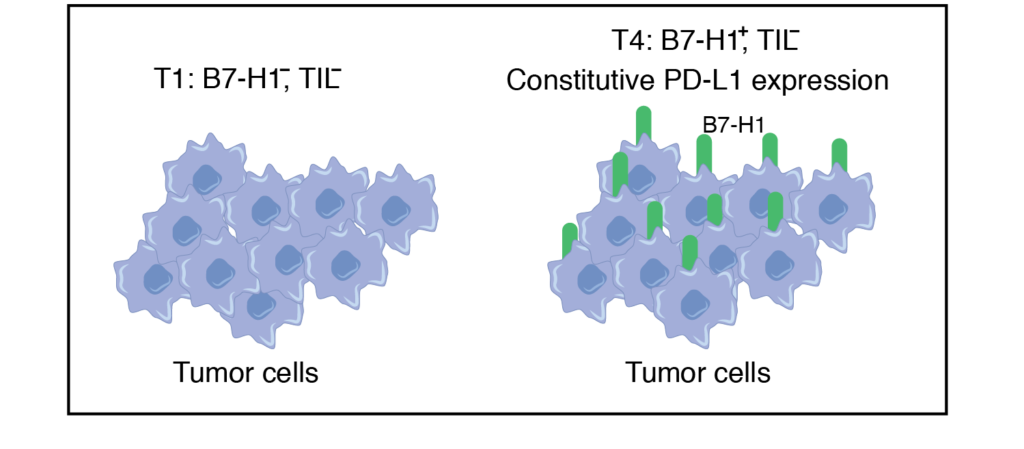
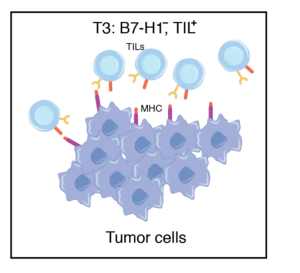
Predicted to respond to anti-PD1 therapy. Tumors containing TILs and other lymphocytes may not respond due to the expression of B7-H1 on tumors
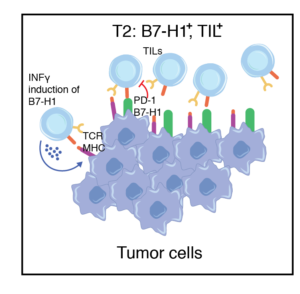
Tumors contain TILs but lack B7-H1 expression, likely owing to the absence of IFNγ production by T effector cells (Teff) and cellular dysfunction.
T4 tumors are similar to T1 except that expression of B7-H1 is present on tumors.

Modules in IO
- Immune 3D can be used to assess factors that increase immune cell migration and infiltration, reduce the production of inhibitory factors and the recruitment of suppressive cells, modulate cellular adhesion, integrins, chemokines and nutrient availability.
- Immune 3D can be used to assess factors that increase immune cell migration and infiltration, reduce the production of inhibitory factors and the recruitment of suppressive cells, modulate cellular adhesion, integrins, chemokines and nutrient availability.
- Our system can be used to assess factors modulating immune cell function, such as immune checkpoints or inhibitor receptor expression.
- Spatial/ temporal relationships between immune cells, tumor cells and surrounding stroma.
- Immune 3D can be set in the presence and absence of immune cells providing a reproducible and affordable platform (compared to in vivo systems) to assess the impact of specific compounds on the tumor microenvironment and the relative contribution of the immune system.
- Importantly, the impact of specific compounds on human tumors and human immune cells can be observed and characterized.
- Immune 3D can be used in combination with immune suppressive agents such as chemotherapy, pharmacological inhibitors, as well as with cells that have been genetically manipulated in order to elucidate the mechanisms impacting anti-tumor immunity.
- Our system provides a novel platform to assess synthetic lethality and the impact of targeting defective DNA repair in cancer cells on anti-tumor immunity.
